Lead author: Seth J Seidman, MS, research electrical engineer and EMC program advisor, Center for Devices and Radiological Health (CDRH), US Food and Drug Administration (FDA)
Supporting authors: Zane Arp, Brian Beard, Joshua Guag, Office of Science and Engineering Labs, CDRH
Introduction
Implantable pacemakers and implantable cardioverter-defibrillators (ICDs) are designed to include a “magnet mode” feature. This feature is designed to be used when a patient is undergoing a procedure where electromagnetic interference (EMI) is possible, or (for ICDs) any time suspension of tachycardia detection and therapy is needed. Magnet mode in a pacemaker is used to force asynchronous pacing and gain control of inappropriate tracking or rate response operation. Magnet mode in an ICD can disable tachycardia detections but does not generally affect pacing functionality. A simple doughnut magnet, of approximately 90 gauss (G) strength, is the standard for triggering the magnet mode feature.
However, this feature is also susceptible to being triggered from any strong static magnetic field. Historically, magnets that are strong enough to trigger magnet mode in a pacemaker or ICD were very large or identifiable (e.g., stereo speakers, electric motors in cordless tools). Pacemaker patients were relatively unaffected from these magnets by maintaining some separation distance since the static magnetic field decays exponentially with distance.
However, with the proliferation of small rare-earth magnets (e.g., neodymium), the environment is changing. These magnets are small enough to be and are being increasingly used inside of headphones, locks for doors, and small phone speakers, as well as emerging technologies including e-cigarettes and smart watches (and accessories).
For example, the MagSafe feature in the iPhone 12 series of phones uses a strong rare-earth magnet to align the phone for wireless charging. A publication in Heart Rhythm demonstrates an iPhone 12 triggering the magnet mode of a Medtronic ICD.
While the iPhone 12 is just another application with a strong magnet, it is a concern for patients because the phone can get very close to the implantable device (e.g., placing the phone in a front shirt or jacket pocket). ICDs in magnet mode will not deliver lifesaving shocks or antitachycardia pacing therapy, which can be life-threatening to the patient if a dangerous abnormal heart rhythm were to occur. Patients might hear an electronic tone indicating that the device is entering magnet mode, depending on the ICD make and model. When a pacemaker enters magnet mode, the device continuously paces without sensing the patient’s own heart rhythm, which can result in symptoms (such as irregular heartbeats), abnormal heart rhythms, or, rarely, more serious patient harm. Implantable pacemakers and ICDs often include a warning to keep phones at least 6 inches away from the implanted medical device. Following Greenberg et al, Apple published a notice on its website also cautioning users to keep phones at least 6 inches away from the implanted medical device.
Magnetic interference (entering magnet mode) is very different than typical EMI to pacemakers and ICDs, where the implantable device mistakenly senses an emitter as a heartbeat. During magnetic interference the implantable device is operating as intended, recognizing a strong magnet, and reverting to magnet mode. Napp et al provide an overview of different types of EMI to implantable devices. The likelihood of magnetic interference varies depending on the type of implant and proximity to any consumer electronic devices that may create magnetic interference, including cell phones and smart watches. Implantable pacemakers and ICDs that meet the specifications from the FDA-recognized ISO 14117 standard are tested to ensure magnet mode will not be triggered by static magnetic fields below 1 mT (10 G).
The purpose of this study is to determine the minimum separation distance between consumer electronic devices that may create magnetic interference, including cell phones and smart watches, and implantable pacemakers and ICDs where magnet mode can be triggered. This study focuses on the iPhone 12 and Apple Watch 6, but the results could be broadly applicable to any consumer electronic devices that may create magnetic interference, including other cell phones and smart watches.
Methods
The static magnetic fields of the iPhone 12 models and Apple Watch were measured. The phones and watches included in this study are iPhone 12 Pro Max, iPhone 12 Pro, iPhone 12, iPhone 12 Mini, and Apple Watch 6. All phones and watches were fully charged prior to the measurement and disconnected from charging cables during all measurements. All wireless communications were turned off, and the phones and watches were put in airplane mode to minimize any potential EMI to our measuring equipment.
The measurement was made using an FW Bell 5180 Gauss Meter with STD18-0404 Transverse probe (unidirectional probe) (OECO LLC, Portland, OR). The probe was zeroed at the beginning of measurement using zero gauss chamber. The noise floor after zeroing was between 0 and -0.6 G. The Speag DASY52 NEO system (SPEAG, Zurich, Switzerland) was used for accurate and repeatable positioning of the probe.
The phones and watches were placed screen face down on a nonconductive surface. For the Apple Watch 6, an additional measurement was made with the watch placed screen face up (see Figure 1). The transverse probe measured X, Y, and Z components of the static magnetic field (see Figure 2). Measurements for each phone were made at 1 cm resolution in a series of XY planes over each phone. All measurements for the Apple Watch 6 were made with 5 mm resolution. The XY measurement planes were at 1, 11, 21, and 31 mm above the top surface of the phones and watches (e.g., camera for iPhones). For the iPhone 12 Pro Max Model, an additional measurement was made at 1 mm above the surface of the main body (excluding camera side of the phone).
Figure 1: Static magnetic field measurement for (left) iPhone, (middle) back side of Apple Watch, (right) screen side of Apple Watch.

Figure 2: Static magnetic field measurement for (left) X-component, (middle) Y-component, and (right) Z-component.
Results
The maximum static magnetic field was measured 1 mm above the back side of the Apple Watch 6. For measurements close to the phones and watches (i.e., 1 mm), the maximum static magnetic field was measured near the alignment magnet. Additionally, we measured high magnetic fields at locations near the camera. For distances of 11 mm or more, the maximum static magnetic field was measured near the center of the MagSafe magnet.
As expected, the Z-component (perpendicular to the phone or watch surface) was the dominant static magnetic field component. Tables 1 and 2 show the maximum static magnetic field measured for the Z-component and for vector magnitude, respectively, at each XY plane for each phone and watch. Figure 3 shows static field measurement for the iPhone 12 Pro. This includes X, Y, Z components and vector magnitude at different XY measurement planes. Static magnetic field measurements from other iPhone models show similar field distribution.
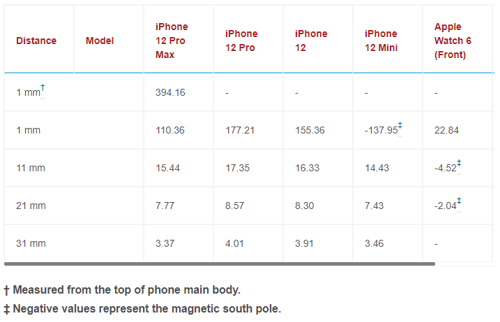
Table 1: Maximum static magnetic field measured for Z-component for each phone and watch at each measurement plane (in gauss)
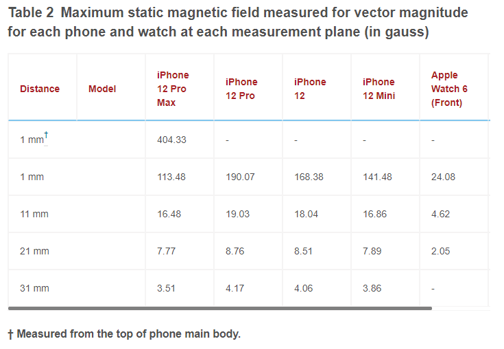
Table 2: Maximum static magnetic field measured for vector magnitude for each phone and watch at each measurement plane (in gauss)
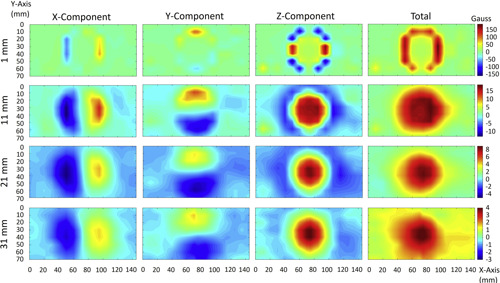
Figure 3: Static magnetic field measurement for iPhone 12 Pro. Each row shows static magnetic field measured in gauss at each measurement plane (XY plane): 1, 11, 21, and 31 mm above the top surface of the iPhone 12 Pro. Each column shows X, Y, and Z components of the static magnetic field, as shown in Figure 2, and the right-most column shows the vector magnitude for each measurement plane.
Limitations
The magnetic field distribution changes rapidly close to the magnet, so a smaller measurement resolution could result in a higher maximum static magnetic field, especially at 1 mm separation distance.
This study was primarily aimed at determining the static magnetic field strength coming from iPhone 12 models and an Apple Watch 6 and comparing that to the levels that trigger magnet mode in implantable pacemakers and ICDs. The measurement data presented can be used to determine if other medical devices susceptible to strong static magnetic fields are affected (e.g., neurostimulators with magnet modes, infusion pumps, cochlear implants).
Discussion
All iPhone 12 and Apple Watch 6 models tested have static magnetic fields significantly greater than 10 G in close proximity (1–11 mm), which attenuates to below 10 G between 11 and 20 mm. If placed within close proximity, the fields are high enough to place implanted cardiac devices into magnet mode. When a separation distance of 6 inches is maintained between an iPhone 12 (or Apple Watch 6), the implantable device should not be put into magnet mode.
The FDA recommends patients keep any consumer electronic devices that may create magnetic interference, including cell phones and smart watches, at least 6 inches away from implanted medical devices, in particular cardiac defibrillators. Many implanted medical devices have FDA-approved information written for patients (patient labeling), which cautions patients to keep all cell phones and smart watches at least 6 inches from the implanted medical device.
People with implanted medical devices may want to take some simple precautions, including:
- Keep the consumer electronics, such as certain cell phones and smart watches, 6 inches away from implanted medical devices.
- Do not carry consumer electronics in a pocket over the medical device.
- Check your device using your home monitoring system if you have one.
- Talk to your health care provider if you are experiencing any symptoms or have questions regarding magnets in consumer electronics and implanted medical devices.
Conclusion
The findings of this study support the FDA recommendation that patients keep any consumer electronic devices that may create magnetic interference, including cell phones and smart watches, at least 6 inches away from implanted medical devices, in particular pacemakers and cardiac defibrillators.

